The DiaFocus system – which is built on top of CARP – has been documented and published as part of a feasibility study. The paper is published in the ACM Transaction on Computing in Healthcare as:
Bardram, J. E., Cramer-Petersen, C., Maxhuni, A., Christensen, M. V., Bækgaard, P., Persson, D. R., … & Jones, A. (2022). DiaFocus: A Personal Health Technology for Adaptive Assessment in Long-Term Management of Type 2 Diabetes. ACM Transactions on Computing for Healthcare.
The paper describes the
- The basic concept of “Adaptive Assessment”
- The user-centered design process which led to the DiaFocus app and system
- The software architecture and implementation of DiaFocus
- A small (N=12) feasibility study over six weeks
Based on this, the paper discusses the usefulness of using digital phenotyping technology, mobile sensing, and adaptive assessment for treating and caring for Type 2 Diabetes (T2D).
In the paper, the overall architecture of DiaFocus is described as illustrated in Fig. 1 below.
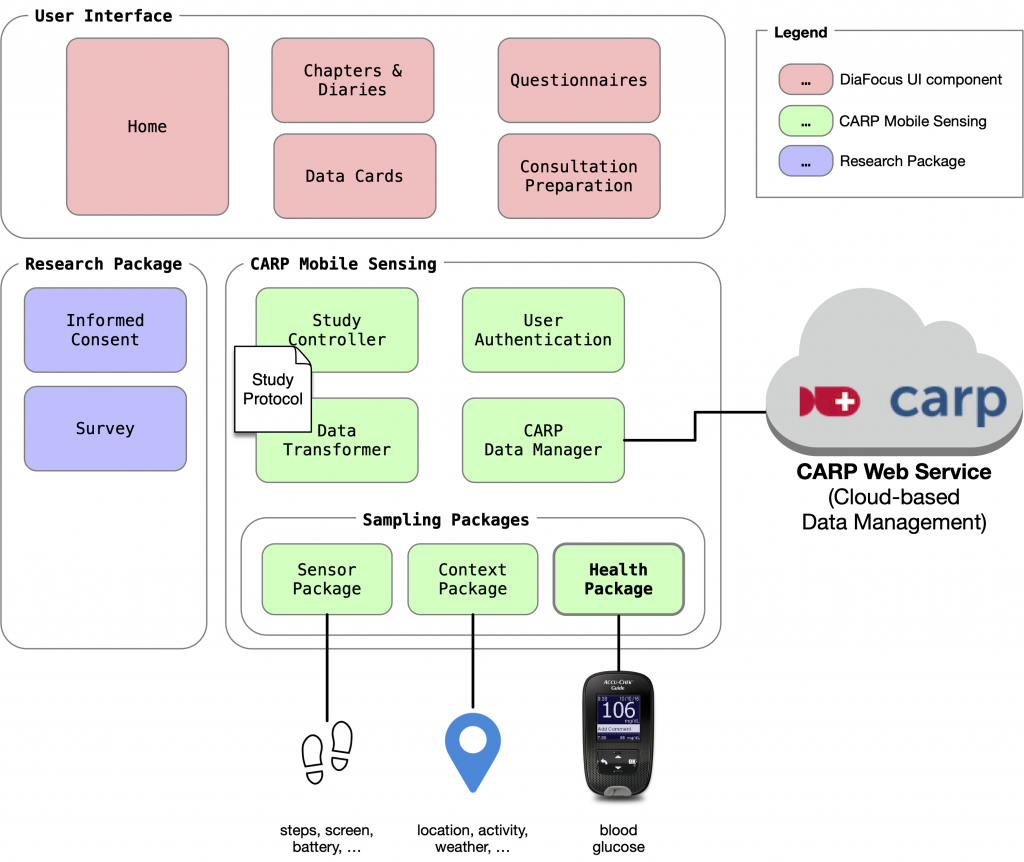
An essential part of DiaFocus was using CAMS for device integration to glucose monitors as device sampling packages and using Apple Health / Google Fit via the health Flutter plugin and the health sampling package.
During the study, the DiaFocus app collected a wide range of both sensing and patient-reported data, as shown in Fig. 2 below.
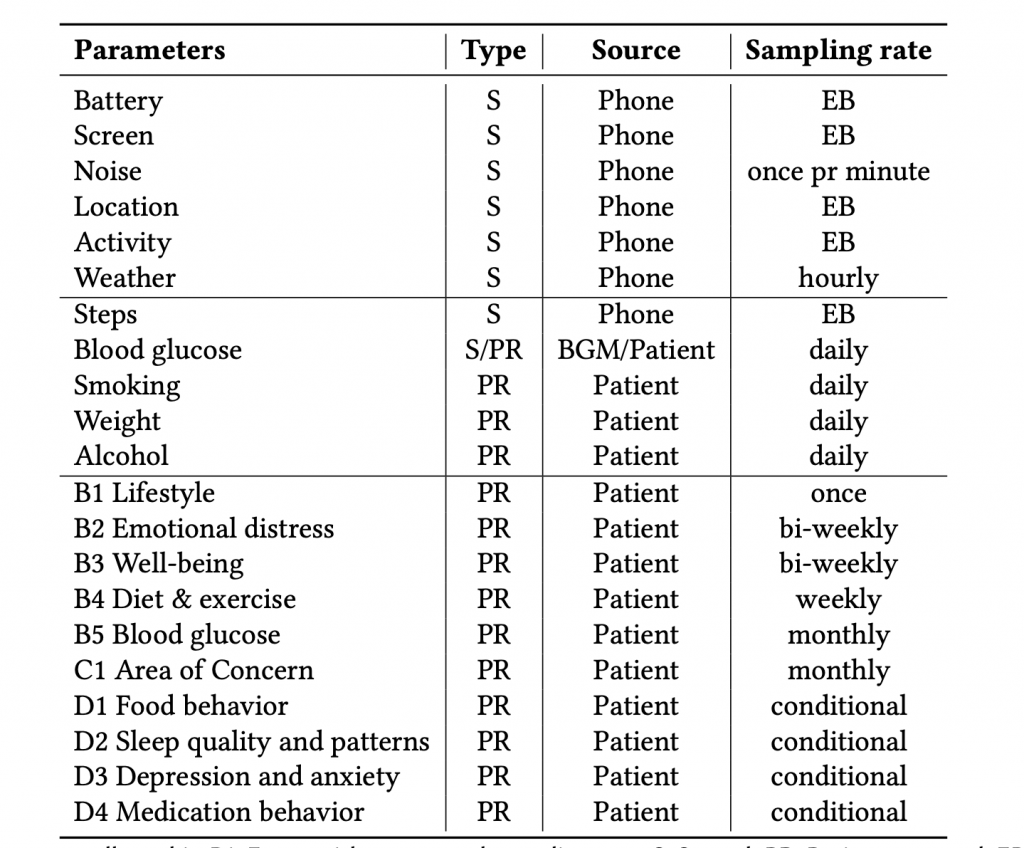
Fig. 3 shows the number of data points collected during the feasibility study. More than 2600 data points were collected over five months. As shown in Table 1, data collection includes both sensed (S) and patient-reported (PR) data. Automatically sensed data include step count and device and battery characteristics. Patient-reported data include surveys (on lifestyle, well-being, emotional distress, sleep quality, depression, anxiety, etc.) and self-reported glucose measures, weight, alcohol intake, and the number of cigarettes smoked.
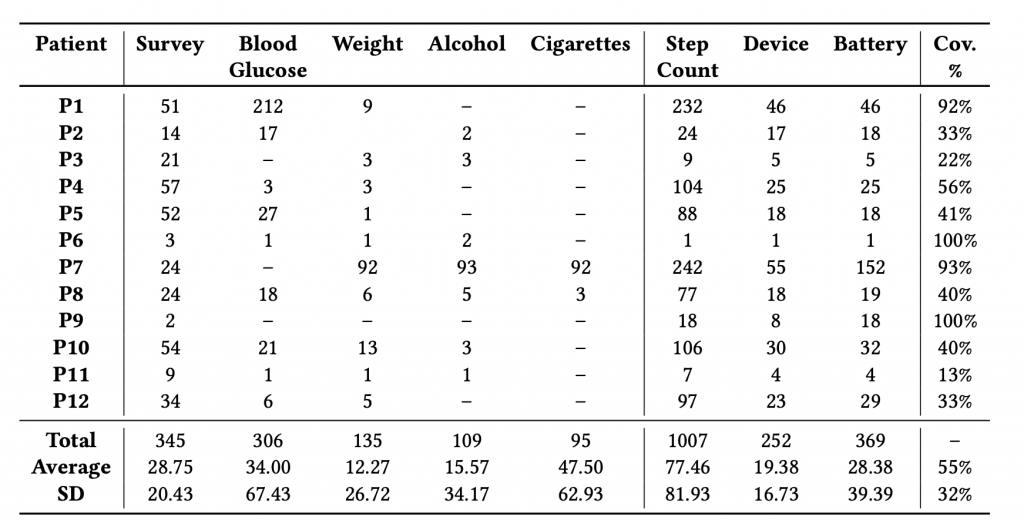
One interesting finding was that all participants, except one, were using iPhones. Hence, sampling coverage is highly affected by the fact that iOS does not allow for sampling in the background.
Based on this study, we conclude that:
The patients found the DiaFocus approach and system useful and usable for diabetes management. Most patients would use such a system, if available as part of their treatment. Analysis of the collected data shows that mobile sensing is feasible for longitudinal ambulatory assessment of T2D, and helped identify the most appropriate target users being early diagnosed and technically literate T2D patients.
Please see the paper for much more details and a discussion on the use of mobile sensing in diabetes treatment.
DiaFocus has been part of a clinical feasibility study, and we’re in the process of analyzing this data. More on this will follow once that study is published.